General Research Approach
Our research goal is to rapidly and efficiently synthesize complex organic molecules of interest and utility while creating powerful synthetic methodologies. For example, transformations using transition-metal catalysts that stabilize carbene and ylide-like intermediates are sought to form densely functionalized bonds and to do so with chemo- and stereoselectivity. Furthermore, cascade reactions allow for a variety of bond forming and bond breaking events to occur in one reaction setup, thus providing significant chemical transformation with high efficiency.
Target molecules are principally complex natural products that exhibit a challenging architecture and intriguing biological properties. Our strategy for synthetic planning incorporates efficiency and practicality so the result of our efforts is a synthesis that can be utilized for reliable access to the natural product target and analogues of said target. Other molecular targets include recognition motifs for directed chemical reactions or molecular biology applications. These compounds have value for therapeutic target identification, pharmaceutical development, protein sequencing, and chemical biology.
Training in the lab includes education in the general fields of organometallics, organocatalysis, synthetic organic chemistry, reaction development, mechanism elucidation, natural product chemistry, structural elucidation, scientific writing, and other skills necessary for the practice of chemistry in an academic or commercial environment.
Natural Product Synthesis
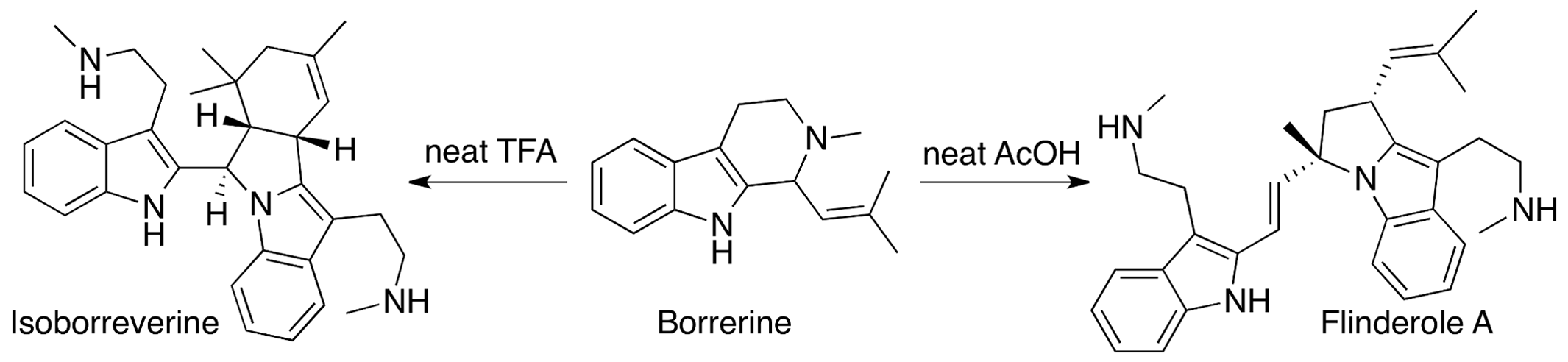
We have achieved a three-step biomimetic synthesis of the antimalarial alkaloids isolated from the genus Flindersia. Thus, tryptamine is transformed in two steps to the natural product borrerine, which is then treated with acid. The resulting cascade sequence results in the selective formation of flinderole A and desmethylflinderole C or isoborreverine depending on the conditions used. If borrerine is first treated with a methylating agent and then acid, flinderole B, flinderole C, and dimethylisoborreverine can be obtained.
Methods Development
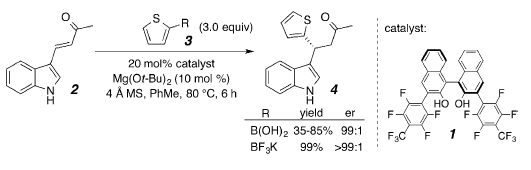
We have developed an enantioselective conjugate addition of vinylboronic acids to ß-indolyl enones. Though these enones are deactivated by the electron-rich heterocycle, and indoles are well known for their ease of acidic and oxidative decomposition, this approach provides indoles with adjacent stereocenters in high yields and excellent enantioselectivities. Further catalyst development has now enabled the transformation to efficiently occur with a wide rage of heteroaryl-appended enones, allowing us to build a library of alpha-chiral heterocyclic compounds.
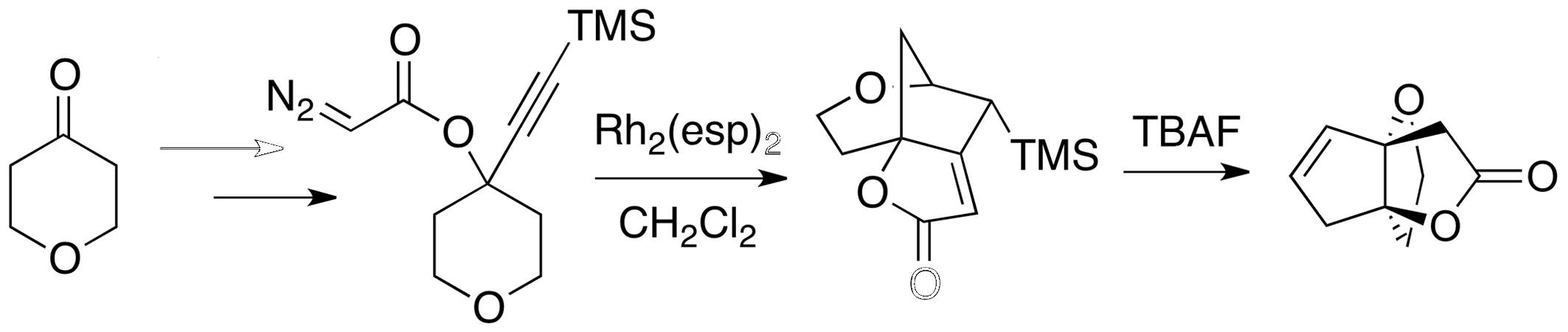
Our work with carbenes has afforded a powerful cascade reaction that builds bridged bicyclic motifs common to biologically active natural products. The connectivity and ring size in the product can be controlled and predicted. Heterocyclic products readily rearrange to prepellanes. This strategy is now allowing the synthesis of multiple natural products.

.gif)