|
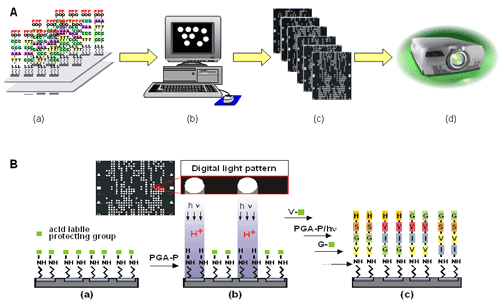
Our laboratory and our collaboration team developed digital photochemistry for parallel synthesis using photogenerated reagents (PGR chemistry). Digital chemistry refers to computer-controlled or programmable chemical reactions. This can be achieved through photogeneration of reaction reagents which allow reactions using conventional building block monomers and solvents. The process of parallel synthesis using our PGR chemistry is illustrated below (Figure 3).
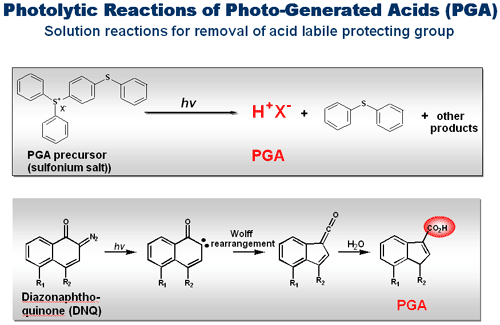
Figure 3. Illustration of parallel combinatorial synthesis using digital photochemistry. In panel A, (a) A list of molecular sequences to be synthesized. (b) This information is processed by computer programs (c) to produce light irradiation patterns that are used to modulate a digital projector for light projection. (d) A projector for projecting the light patterns. In panel B, (a) in a typical synthesis, a surface is functionalized with amino with protection groups. (b) To this surface, photogenerated acid precursor (PGA-P) solution is applied and light is projected to selective reaction sites according to a defined light pattern. Under these conditions, PGA-P is converted into an acid (PGA) which removes the protecting group to expose surface amino groups. (c) When monomers are added to the surface, these molecules are connected to the deprotected amino groups to give coupling products. Repeating reaction cycles of light directed PGA deprotection and coupling produce final product ?addressable molecular microarrays of user defined sequences. Using this digital chemistry, we have established a robust process for DNA synthesis on microfluidic chips (see Microfluidic platform) and have commercialized DNA chips (XeoChipTM) products (Xeotron Co.). On this platform, various DNA and RNA oligonucleotides with modifications can be conveniently synthesized. A few examples of these include chips of RNA, phosphothioate-oligonucleotides, methylated oligonucleotides, and LNA (Wengel and co-workers (1999) J. Chem. Soc. Perkin Trans. 1, 2543-2551). We further demonstrate that the chip is a miniaturized synthesizer for oligonucleotides and is an efficient and low cost means of obtaining oligonucleotides for multiplex reactions such as cloning using inserts and gene synthesis. In addition to oligonucleotide parallel synthesis, we have extended the digital chemistry to peptide parallel synthesis using PGA. The applications of these high density peptide chips include epitope screening using specific recognition antibodies (antibody profiling) and kinase/phosphatase/protease activity profiling and probing substrate specificity. The availability of a large number of amino acid analogs for synthesis makes it possible to create diverse molecular libraries of peptidomimetics compounds, peptoid and other families of peptide bond containing compounds on chip. These are powerful tools for high throughput drug discoveries. In summary, the development of the digital chemistry is a key step towards miniaturization and automation of parallel synthesis. This method integrates multiple steps into a programmable process. We anticipate that this method increasingly finds more applications in the array synthesis of a variety of organic and biomolecules. A key component in our digital chemistry is photogenerated reagent (PGR) which is formed under photolytic reaction conditions (see Digital Chemistry). In fact, photogenerated acids (PGAs), a type of PGR, have been used for many years as polymerization initiators and as photoresist processing reagents in printing and microelectronic industries. Knowing the properties of PGA compounds, we explore the idea of in solution photogeneration of acids and use the PGA for conventional reactions. If we can control the generation of reaction reagents, the synthesis can be controlled as desired. This method opens the door for parallel synthesis of not only DNA but also other types of molecules. A typical PGA reaction is shown in Figure 4. As seen in the figure, a PGR should contain a chromophore moiety that responds to light and triggers photolytic reaction. The presence of an activation sensitizer of PGR sometimes is needed for efficient reactions. Figure 5 shows another example of PGA-P and its photolytic reaction to form a PGA (a carboxylic acid in this case).
Figure 4. Illustration of a photogenerated acid (PGA) reaction. (A) Photolytic reaction of a sulfonium salt to produce protic acid (H+) in methylene chloride. (B) The PGA removes the DMT protecting group on an OH group. (C) The PGA formation only occurs at light irradiated reaction sites. Therefore, only these sites are ‘activated?for next step reaction
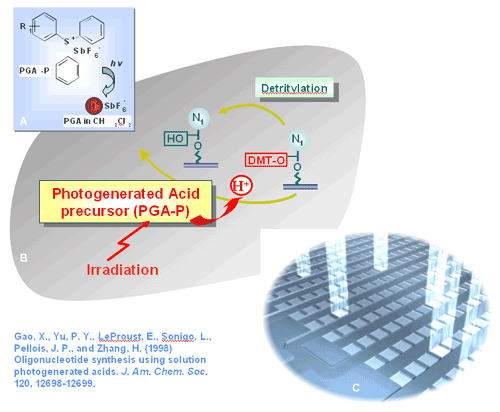
Figure 5. A classic reaction of a PGA-P under light irradiation and the formation of PGA (indenecarboxylic acid) (Ito, H. (1997) IBM J. Research vol 41). Our research in PGR chemistry has led to the development of efficient PGA reaction conditions for oligonucleotides and peptide synthesis. Our interests lie in the areas of the development and applications of PGR such as photogenerated bases (PGB) in high throughput synthesis of diverse categories of molecules.
|
||
| UofH | Chemistry | Biology&Biochemistry | ACS | NCBI | PDB | CSCPDB | SeqTools | NMRTools Login | ||